Background:Acute Myeloid leukemia (AML) and High-risk Myelodysplastic Syndrome (MDS) are two aggressive myeloid neoplasms characterized by dismal outcomes in patients not suitable for intensive treatment. During the last decades, hypomethylating agents (HMA) represented a valuable treatment option for AML and MDS, particularly in older or unfit patients. However, the limited efficacy and high-costs concerns in developing countries limited its general use. Azacytidine is the most representative and available HMA in limited-sources facilities. We aimed to explore the efficacy of azacytidine as frontline treatment in terms of event-free (EFS) and overall survival (OS) for newly diagnosed patients with AML non-candidates to intensive chemotherapy and MDS in Peru.
Methods: We reviewed medical records of patients diagnosed and managed for AML at three private cancer institutions in Lima-Peru (Oncosalud, Clinica Delgado and Clinica Angloamericana) between 2010 and 2019. Cytogenetics and molecular markers were not retrospectively available during data obtention. Clinical characteristics, laboratory data and outcomes were evaluated for patients with AML and MDS who received at least 1 cycle of AZA as frontline treatment. AZA was given at accumulated dose of 75mg/m2/daily for 7 days every 4 weeks, however further dose reductions were allowed by physician criteria. Best responses were assessed according to Leukemia.net consensus, but summarize as complete remission (CR), partial response (PR) and stable disease (SD). Survival curves (event-free and overall survival) were estimated using the Kaplan-Meier method and compared with the Log-rank test.
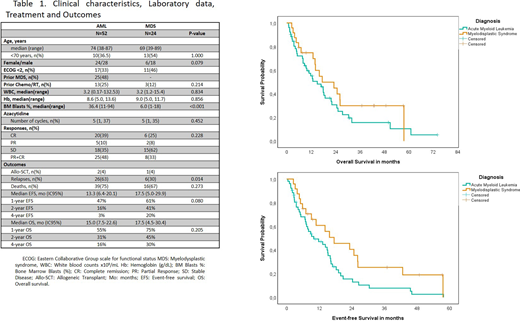
Results:A total of 76 patients were included and had sufficient data for analysis. Fifty-two cases had AML and 24 MDS. Clinical characteristics, Laboratory data, Treatment and Outcomes were summarized in Table 1. Median ages for AML was 74 and 69 for MDS, 37% and 54% had less than 70 years in AML and MDS, respectively. Female/male ratio was 1:1 for AML and 1:3 for MDS. One-third of AML cases and 48% of MDS had an ECOG performance status ≤1. Twenty-five (48%) of AML patients had Secondary AML to MDS, and 25% had prior cancer treatment. Median number of cycles of AZA was 5 for both groups. A high composite CR rates were seen for AML (39%) and MDS (25%), PR were 10% and 8% for AML and MDS, respectively. The majority of MDS cases reached stable disease (62%), and 35% in AML. Three patients underwent to allo-transplant (2 AML and 1 MDS). Relapsed rates were more frequent in AML cases (AML 63% vs. 30%), however, deaths were similar in both groups. At a median follow-up of 4-years, the EFS was 3% for AML and 20% for MDS, and OS was 16% and 30% for AML and MDS patients, respectively.
Conclusions:
Azacytidine represents an effective and accessible therapy for AML non-candidates to intensive chemotherapy and MDS in limited-sources settings. Access to novel effective therapies must be warranted for these highly aggressive myeloid neoplasms in developing countries.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal